

 GPC is a technique to separate components based on different molecular weight sizes. It separates using porous
polymer matrix whose pores are filled with mobile phase (solvent).
GPC is a technique to separate components based on different molecular weight sizes. It separates using porous
polymer matrix whose pores are filled with mobile phase (solvent).
There are two types of GPC which are aqueous and organic. For aqueous, the mobile phase is water and as for organic
the solvent used either DMF, THF or DMSO.
The range of molecular weight that can be detected are from hundreds to millions.
The flow of the mobile phase hence will cause larger molecules to pass through the column unhindered,
without penetrating the gel matrix, whereas smaller molecules will be retarded according to their penetration of the gel.
From this simple mechanism, the molecular weight spectrum of samples will be recorded according to time.
This test follows the standard D5296-11 entitled Standard Test Method for Molecular Weight Averages and Molecular Weight Distribution
of polystyrene by High Performance Size Exclusion Chromatography
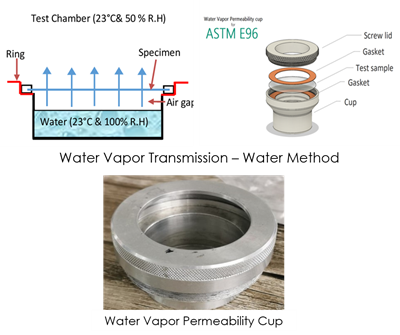 ASTM E96/E96M-16 – Standard Test Methods for Water Vapor Transmission of Materials addresses a testing procedure for
determining the water vapor permeability through some porous materials, such as paper, plastic films, fiberboards, gypsum,
plaster products, wood products, and plastics, , which can be incredibly useful for knowing the potential risks posed on the
materials in different humidity levels. This measurement also essential for compiling a methodical comprehension of materials
desired for use. The standard’s testing method offered is by Water Method. The test specimen is sealed to a test cup containing
distilled water, and the assembly is placed in a controlled atmosphere. Periodic weighing are used to assess rate of water vapor
movement through the specimen from the water.
ASTM E96/E96M-16 – Standard Test Methods for Water Vapor Transmission of Materials addresses a testing procedure for
determining the water vapor permeability through some porous materials, such as paper, plastic films, fiberboards, gypsum,
plaster products, wood products, and plastics, , which can be incredibly useful for knowing the potential risks posed on the
materials in different humidity levels. This measurement also essential for compiling a methodical comprehension of materials
desired for use. The standard’s testing method offered is by Water Method. The test specimen is sealed to a test cup containing
distilled water, and the assembly is placed in a controlled atmosphere. Periodic weighing are used to assess rate of water vapor
movement through the specimen from the water.
 * Temperature Humidity Chamber also called Climatic Chamber, can simulate various temperature and humidity environment for high
low temperature operation & storage, temperature cycling, damp heat, low temperature & humidity.
* Temperature Humidity Chamber also called Climatic Chamber, can simulate various temperature and humidity environment for high
low temperature operation & storage, temperature cycling, damp heat, low temperature & humidity.
* The simulation condition is required to test specimen's performance and reliability on biological items, industrial products,
electronic devices and components.
* This Test Chamber can meet wide range from high temp. up to +150℃ to low temp. down to -40℃. While humidity range can be 20%
to 98%.
* List of testing related to the usage of the chamber are ageing test, stability test, shelf life and water vapour transmission
test.
Cell attachment is the initial step in a cascade of cell–biomaterial interactions and is important to cellular processes such as cell guidance, proliferation, and differentiation. Cell–surface interactions are fundamental to understanding cell behaviour and provide an important means to control and quantify cell activity. We provide cell attachment study by fixing the cells onto the materials.
* To perform on media equipment and product.
* To measure the number of living organism on surface prior to final sterilization and use.
* ISO 11737 - This document provides guidance on the microbial computation and characterization of eligible microorganism populations on or in products, components, raw materials or healthcare packages.

* Observation at any effect on, Interaction with, or response from living tissue.
* Service provided:
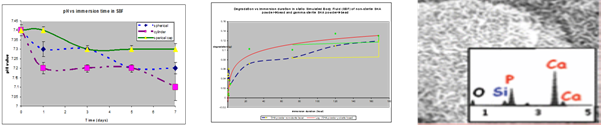
- In vitro apatite forming ability in SBF or PBS
- Evaluation of ion concentrations of Ca ion detection
- Measurement of pH and evaluation of mass loss.
 * Thermogravimetric analyzer (TGA) is an equipment for conducting materials characterization through thermogravimetric analysis.
TGA measures the amount and the rate of weight change of a material with respect to temperature or time in controlled environments.
* Thermogravimetric analyzer (TGA) is an equipment for conducting materials characterization through thermogravimetric analysis.
TGA measures the amount and the rate of weight change of a material with respect to temperature or time in controlled environments.
* A TGA can be used to characterize materials through analysis of characteristic decomposition patterns and thermal stability in
both quality control and research applications on industrial products such as pharmaceuticals, polymers, food, metals and alloys.
It is an useful technique for the study of polymeric materials including thermoplastics, thermosets, composites, coatings, plastic
films, fibers, and paints.
* Our TGA is operated based on ASTM E1131-08 (Reapproved 2014) Standard Test Method for Compositional Analysis by Thermogravimetry,
and guidelines in International Standard ISO11358-1:2014 Plastics-Thermogravimetry (TG) of polymers. Both are applicable to liquids
and solids. The temperature range of test is typically room temperature to 1000°C. Composition between 1 and 100 weight % of
individual components may be determined. Sample is heated in a given environment (air, N2, CO2, He, Ar, etc.) at controlled rate.
 * Oxygen transmission rate is the steady state rate of transmission of oxygen gas through the plastics in the form of film,
sheeting, laminates, plastic coated papers or fabrics.
* Oxygen transmission rate is the steady state rate of transmission of oxygen gas through the plastics in the form of film,
sheeting, laminates, plastic coated papers or fabrics.
* Our Oxygen Permeability Analyzer is Systech Illinois 8501. The standard test conditions are 23⁰C (73⁰F) and 0% RH where the
values are expressed in cc/m2/24hr in metric units.
* ASTM D 3985-02: Standard test method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric
Sensor.
* The behavior of packaging permeability and barrier properties can be determined through this test. Food packaging performances
can be checked and importance to preserve the food quality and extend the food shelf life. Other than that, by discover the
optimum behavior of packaging film. We can reach the best engineering solution during processing.
 Imaging by VPSEM has the advantages over conventional Scanning Electron Microscope by providing operational in low vacuum mode in
which the pressure can be adjusted in the sample chamber until the artefact of electron charging is removed from images.
The surface of non-conductive specimen can be imaged without conductive coating prior image detection. This opens the opportunity
to visualize polymers, biological samples and museum historical artefact samples. Low vacuum mode also capable to freeze-dry samples
for viewing. The technique works well on hydrated samples that acquire basic structural integrity such as botanical and zoological tissues.
Imaging by VPSEM has the advantages over conventional Scanning Electron Microscope by providing operational in low vacuum mode in
which the pressure can be adjusted in the sample chamber until the artefact of electron charging is removed from images.
The surface of non-conductive specimen can be imaged without conductive coating prior image detection. This opens the opportunity
to visualize polymers, biological samples and museum historical artefact samples. Low vacuum mode also capable to freeze-dry samples
for viewing. The technique works well on hydrated samples that acquire basic structural integrity such as botanical and zoological tissues.
 * Polymer testing of medical devices is integral to product development, improvement, and failure analysis investigations.
With increased use of polymer materials in implantable medical devices, the characterization of degradation products is a main
particular interest for both in risk assessment and regulatory perspectives. The analysis may include the interrogation of the
terminal degradation products of a material, as well as the intermediate degradation products that release prior to complete
dissolution or breakdown of the material.
* Polymer testing of medical devices is integral to product development, improvement, and failure analysis investigations.
With increased use of polymer materials in implantable medical devices, the characterization of degradation products is a main
particular interest for both in risk assessment and regulatory perspectives. The analysis may include the interrogation of the
terminal degradation products of a material, as well as the intermediate degradation products that release prior to complete
dissolution or breakdown of the material.
* With an experienced eye on current regulatory specifications (ISO 10993-13:2010 Biological evaluation of medical devices
Part 13: Identification and quantification of degradation products from polymeric medical devices), our laboratory will assist
you with the structuring studies in order to characterize what your biodegradable polymer is breaking down into, as well as the
concentration of these eluted products as a function of the material lifetime. These studies typically require careful
consideration of the material chemistry and end-use biological environment for the purpose of selecting the in vitro degradation
conditions.
* Our polymer degradation analysis and studies shall include degradation product analysis (identification and quantitation),
polymer degradation experimental design and extractable and leachable testing evaluation of functional material properties during
degradation. Testing that may be employed to study the effect of degradation process on the polymeric may involve GPC, SEM, FTIR,
TGA and UV-vis.
In order to ensure the safety profiles of the resorbable ceramic based medical devices, confirmation through the degradation and
dissolution are performed to confirm that the ceramic products are free from processing contaminants and residuals, such as
machining oils and cutting fluids as well as self-leaching ions. Degradation test is carried out by performing agitation etching
endurance test for 120 h using extreme (pH 3.0) and simulation (pH 7.5) solutions representing worst case and normal body pH
level, respectively. Test shall be executed at temperature of 37 OC which represented feverish body condition with guidance
stipulated by ISO 10993 part 14. Dissolution shall be checked on the produced retantate and filtrate and the integrity of the
tested ceramic products are measured by the criteria below:
- Through microscopic imaging for microstructure segregation determination using ISO 3310.
- Purity criteria of the tested ceramic is also obtained through microstructural analysis by guidance of ISO 6474 using x-ray diffractometer.
- Density parameters of the ceramic scaffold are tested for true density, apparent density and apparent porosity following ISO 5017. On the other hand, ceramic powder is tested for it bulk density.
- ASTM D4780 is use as direction for surface area analysis through Brunauer-Emmett-Teller analyser
- Chemical analysis determination on the leached ions is accomplished by Inductively-Coupled Plasma spectrometer.
Advanced Materials Testing Laboratory experts are happy to support manufacturers facing the challenge, providing testing and
offering regulatory expertise during the entire medical life cycle.
 * One of the potential health hazards resulting from medical devices may be due to the interactions of their
electrochemically-induced degradation products with the biological system. Therefore, the evaluation of potential degradation
products from metallic materials by methods suitable for testing the electrochemical behavior of these materials is a necessary
step in the biological performance testing of materials.
* One of the potential health hazards resulting from medical devices may be due to the interactions of their
electrochemically-induced degradation products with the biological system. Therefore, the evaluation of potential degradation
products from metallic materials by methods suitable for testing the electrochemical behavior of these materials is a necessary
step in the biological performance testing of materials.
* Corrosion testing determines the resistance of materials to corrosion under certain environmental conditions, including
temperature, humidity and salt water. ICI Biomedical offers a full array of corrosion testing services to ensure the performance
of metals and coatings.
* The corrosion test is based on the ISO 10993-15 Biological Evaluation of Medical Devices, Part 15 - Identification and Quantification
of Degradation Products from Metals and Alloys. The methods deal with identification and quantification of degradation products
from finished metallic medical devices or corresponding material samples finished as ready for clinical use. It is applicable only
to those degradation products generated by chemical alteration of the finished metallic device in an in vitro accelerated
degradation test.